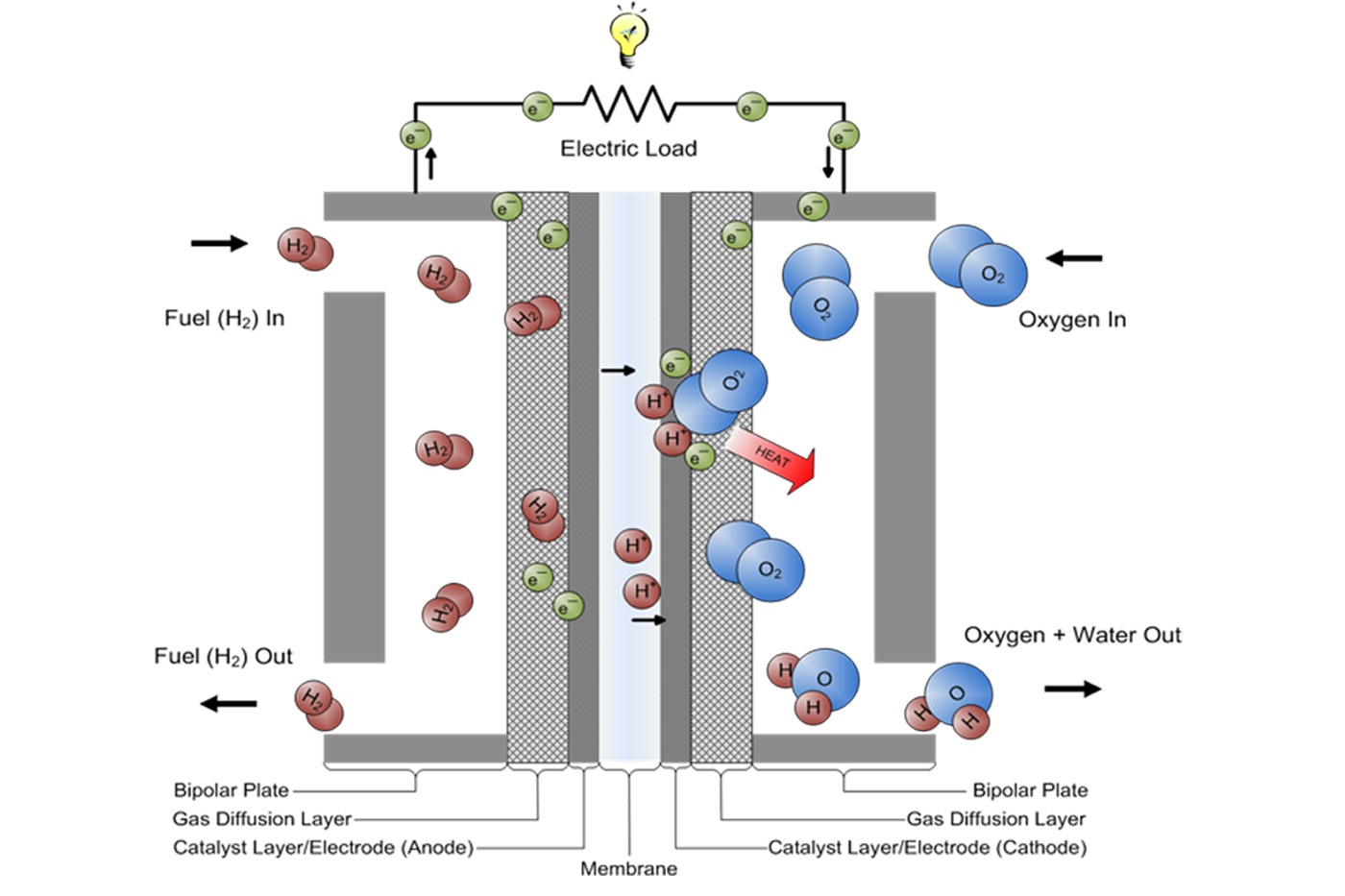

Fuel Cells work via chemical reactions that release energy (electrical charge) in the process. Hydrogen is run through a flow plate on one side of a fuel cell and Oxygen (or atmospheric air) is run through a flow plate on the opposite side. Between these plates is a material that blocks the negatively charged electrons of the H2 molecule but allows the positively charged protons to pass through the PEM. The negative charge is forced to travel through an electrical wire. This is how a fuel cell produces electric current. The negative charge then continues along the wire and is rejoined with the positive charge and Oxygen on the opposite side of the PEM . The end result is pure water.